Abstract
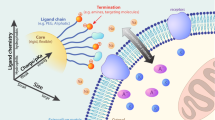
In this research, nanoparticle translocation through cell membrane has been studied and simulated. To this end, gold nanoparticles have been selected as the main carrier of the drug and have been functionalized with some selected ligands. The partial charges of the ligands have been calculated using quantum mechanics based on HF technique with 6-31Gd basis set. To achieve the realistic shape of a drug, the number and arrangement of ligands loaded on the gold nanoparticle have been optimized. After determining the properties such as diffusion coefficient and validating the results with experimental data, a MARTINI coarse-grained mapping of the drugs is created. The coarse-grained model of the drug has been placed in a cellular microenvironment containing a solvent. The cytoplasmic membranes investigated in this study consists of more than 60 types phospholipids similar to animal cell membranes. After minimizing the system and performing procedures for controlling temperature and pressure, ions are added to the system and a potential difference is applied to the membrane and translocation of the drug is studied.
























Similar content being viewed by others
References
Arias JL (2014) Nanotechnology and drug delivery, volume one: nanoplatforms in drug delivery, vol 1. CRC Press, Boca Raton
AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S (2008) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2):279–290
Bardají M, Uznanski P, Amiens C, Chaudret B, Laguna A (2002) Aurophilic complexes as gold atom sources in organic media. Chem Commun 6:598–599
Beik J, Khateri M, Khosravi Z, Kamrava SK, Kooranifar S, Ghaznavi H, Shakeri-Zadeh A (2019) Gold nanoparticles in combinatorial cancer therapy strategies. Coord Chem Rev 387:299–324
Belloni J (1996) Metal nanocolloids. Curr Opin Colloid Interface Sci 1(2):184–196
Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M, MacKerell AD Jr (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 dihedral angles. J Chem Theory Comput 8(9):3257–3273
Busbee BD, Obare SO, Murphy CJ (2003) An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater 15(5):414–416
Carrot G, Valmalette JC, Plummer CJG, Scholz SM, Dutta J, Hofmann H, Hilborn JG (1998) Gold nanoparticle synthesis in graft copolymer micelles. Colloid Polym Sci 276(10):853–859
Chen W, Cai WP, Liang CH, Zhang LD (2001) Synthesis of gold nanoparticles dispersed within pores of mesoporous silica induced by ultrasonic irradiation and its characterization. Mater Res Bull 36(1–2):335–342
Cho EC, Xie J, Wurm PA, Xia Y (2009) Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett 9(3):1080–1084
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Controll Release 148(2):135–146
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
Du Y, Xia L, Jo A, Davis RM, Bissel P, Ehrich MF, Kingston DG (2018) Synthesis and evaluation of doxorubicin-loaded gold nanoparticles for tumor-targeted drug delivery. Bioconjug Chem 29(2):420–430
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60(11):1307–1315
Gomez S, Philippot K, Collière V, Chaudret B, Senocq F, Lecante P (2000) Gold nanoparticles from self-assembled gold (i) amine precursorsElectronic supplementary information (ESI) available: experimental details and full characterization of complexes 2–4, powder XRD spectra of 2 and TEM micrographs of 2 and gold nanoparticles. Chem Commun 19:1945–1946
Ingólfsson HI, Melo MN, Van Eerden FJ, Arnarez C, Lopez CA, Wassenaar TA, Periole X, De Vries AH, Tieleman DP, Marrink SJ (2014a) Lipid organization of the plasma membrane. J Am Chem Soc 136(41):14554–14559
Ingólfsson HI, Melo MN, Van Eerden FJ, Arnarez C, Lopez CA, Wassenaar TA, Periole X, De Vries AH, Tieleman DP, Marrink SJ (2014b) Lipid organization of the plasma membrane. J Am Chem Soc 136(41):14554–14559
Jana NR, Gearheart L, Murphy CJ (2001) Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mater 13(7):2313–2322
Kang B, Mackey MA, El-Sayed MA (2010) Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J Am Chem Soc 132(5):1517–1519
Lattes A, Rico I, De Savignac A, Samii AAZ (1987) Formamide, a water substitute in micelles and microemulsions xxx structural analysis using a diels-alder reaction as a chemical probe. Tetrahedron 43(7):1725–1735
Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C (2013) Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 135(4):1438–1444
Lin J, Alexander-Katz A (2013) Cell membranes open “doors” for cationic nanoparticles/biomolecules: insights into uptake kinetics. ACS Nano 7(12):10799–10808
Lin J, Zhang H, Chen Z, Zheng Y (2010) Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 4(9):5421–5429
Lin JQ, Zheng YG, Zhang HW, Chen Z (2011) A simulation study on nanoscale holes generated by gold nanoparticles on negative lipid bilayers. Langmuir 27(13):8323–8332
Lodish H, Darnell JE, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P (2008) Molecular cell biology. Macmillan, New York
Luedtke WD, Landman U (1998) Structure and thermodynamics of self-assembled monolayers on gold nanocrystallites. J Phys Chem B 102(34):6566–6572
Mahaffy R, Bhatia R, Garrison BJ (1997) Diffusion of a butanethiolate molecule on a Au 111 surface. J Phys Chem B 101(5):771–773
Mandal S, Phadtare S, Sastry M (2005) Interfacing biology with nanoparticles. Curr Appl Phys 5(2):118–127
Mangadlao JD, Wang X, McCleese C, Escamilla M, Ramamurthy G, Wang Z, Govande M, Basilion JP, Burda C (2018) Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano 12(4):3714–3725
Meltzer S, Resch R, Koel BE, Thompson ME, Madhukar A, Requicha AA, Will P (2001) Fabrication of nanostructures by hydroxylamine seeding of gold nanoparticle templates. Langmuir 17(5):1713–1718
Moessmer S, Spatz JP, Moeller M, Aberle T, Schmidt J, Burchard W (2000) Solution behavior of poly (styrene)-B lock-poly (2-vinylpyridine) micelles containing gold nanoparticles. Macromolecules 33(13):4791–4798
Nangia S, Sureshkumar R (2012) Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes. Langmuir 28(51):17666–17671
Obayemi JD, Hu J, Uzonwanne VO, Odusanya OS, Malatesta K, Anuku N, Soboyejo WO (2017) Adhesion of ligand-conjugated biosynthesized magnetite nanoparticles to triple negative breast cancer cells. J Mech Behav Biomed Mater 68:276–286
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751
Pishkenari HN, Yousefi FS, Taghibakhshi A (2018) Determination of surface properties and elastic constants of FCC metals: a comparison among different EAM potentials in thin film and bulk scale. Mater Res Express 6(1):015020
Pol VG, Gedanken A, Calderon-Moreno J (2003) Deposition of gold nanoparticles on silica spheres: a sonochemical approach. Chem Mater 15(5):1111–1118
Ren F, Bhana S, Norman DD, Johnson J, Xu L, Baker DL, Parrill AL, Huang X (2013) Gold nanorods carrying paclitaxel for photothermal-chemotherapy of cancer. Bioconjug Chem 24(3):376–386
Sau TK, Pal A, Jana NR, Wang ZL, Pal T (2001) Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J Nanopart Res 3(4):257–261
Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I (2018) Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci 19(7):1979
Siu SW, Pluhackova K, Böckmann RA (2012) Optimization of the OPLS-AA force field for long hydrocarbons. J Chem Theory Comput 8(4):1459–1470
Tiwari PK, Soo Lee Y (2013) Gene delivery in conjunction with gold nanoparticle and tumor treating electric field. J Appl Phys 114(5):054902
Van Lehn RC, Alexander-Katz A (2013) Structure of mixed-monolayer-protected nanoparticles in aqueous salt solution from atomistic molecular dynamics simulations. J Phys Chem C 117(39):20104–20115
Vinod M, Jayasree RS, Gopchandran KG (2017) Synthesis of pure and biocompatible gold nanoparticles using laser ablation method for SERS and photothermal applications. Curr Appl Phys 17(11):1430–1438
Wang T, Bai J, Jiang X, Nienhaus GU (2012) Cellular uptake of nanoparticles by membrane penetration: a study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano 6(2):1251–1259
Yang K, Ma YQ (2010) Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat Nanotechnol 5(8):579
Yang YSS, Moynihan KD, Bekdemir A, Dichwalkar TM, Noh MM, Watson N, Melo M, Ingram J, Suh H, Ploegh H, Stellacci FR (2019) Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater Sci 7(1):113–124
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Figs. 25, 26, 27, 28, 29 and Tables 8, 9, 10, 11, 12, 13.
Rights and permissions
About this article
Cite this article
Nejat Pishkenari, H., Barzegar, M.R. & Taghibakhshi, A. Study and Simulation of Nanoparticle Translocation Through Cell Membrane. Iran J Sci Technol Trans Mech Eng 45, 939–960 (2021). https://doi.org/10.1007/s40997-019-00326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40997-019-00326-8